The difference between right and left
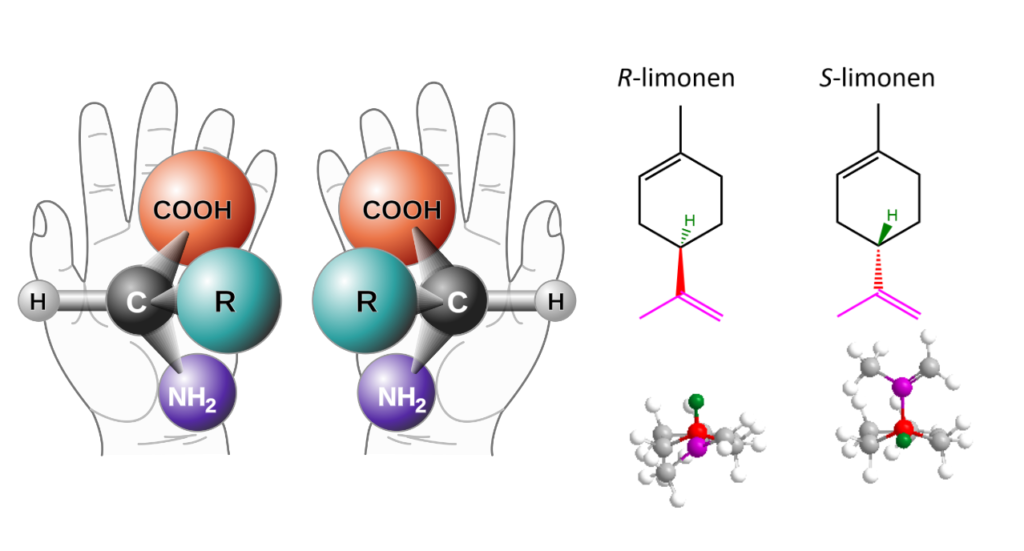
Common feature of many chemical compounds existing in nature is lack of elements of mirror symmetry. If we take a starting molecule A and place it in front of a mirror, obtained mirror image A’ will be spatially different – such as human hands. This feature in chemistry is called chirality. Molecules A and A’ that have identical molecular and structural formulae, but different spatial formulae are called enantiomers. Enantiomers, described as mirror images of each other, interact identically with achiral molecules (which display elements of mirror symmetry). Similarly, we see no difference in the grip of human right or left hand on a simple, straight metal tube. On the other hand, when molecules A and A’ interact with other chiral molecules (e.g. B and B’), spatial structure (i.e. absolute configuration) is critical for the outcome. This phenomenon is readily explained by interaction of human hands of two people – we feel differently grip of other person’s right and left hand. This key feature is utilized by majority of functional biomolecules: nucleic acids, proteins and carbohydrates. Chemistry of life mastered all the interactions through stereochemistry: absolute configuration of building blocks is always the same, chiral secondary structures e.g. helices are right-handed. On the top of that, all biopolymers are monodisperse and monosequential. The pedantry of nature has been a big mystery for chemists around the world. To this day, we have no answers for many fundamental questions: what is the origin of chirality? What were the first chiral compounds? How the chirality evolved? Are there any alternative galaxies where compounds with opposite absolute configuration dominate? There are plenty of similar questions that can be asked, although it takes many years of research to answer even one of them.
The concept of chirality was described by Dutchman Jacobus van ‘t Hoff, who received first ever Nobel Prize in 1901 for his seminal work on spatial structure of organic molecules. One of many ideas he proposed back in XIX century was that enantiomers should exhibit different physicochemical properties when dissolved in an optically active (chiral) solvent. Seemingly such idea appears to be truism, as this should originate from the previous paragraph. However, the facts are strikingly different: until 2019 no one was able to experimentally show that enantiomers do have different properties in a chiral solvent in thermodynamic equilibrium. Therefore we now dive into a story that no one expected to happen, which took place in the laboratories of Eindhoven University of Technology.
Organic molecules posses spatial structure. Carbon atoms form four covalent bonds each. When each constituent at given carbon atom is different, mirror images of the molecules are not superimposable but are like human left and right hand: enantiomers. Nature exploits this property to selectively assign molecules. For example, limonene is chiral molecule and appears in form of two enantiomers. Enantiomer with absolute configuration R occurs in citrus fruit and is responsible for their characteristic smell. On the other hand, enantiomer with absolute configuration S is rare and has smell of turpentine. This difference is explained by chirality of receptor molecules in human nose. Chiral receptor molecules interact differently with enantiomer S than R thereby affecting the signaling process.
Rebellion in a flask
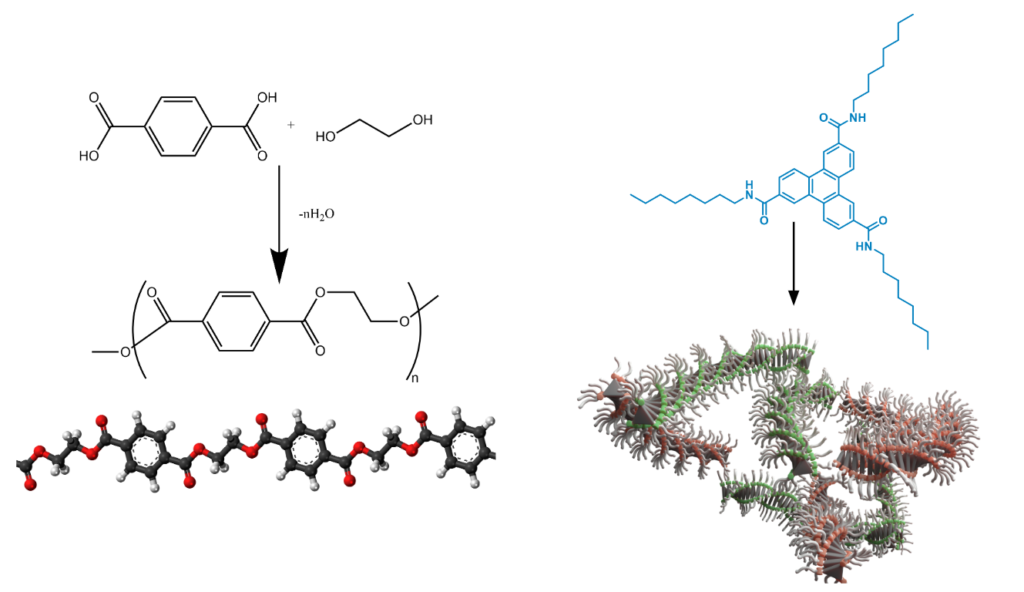
During my PhD time I investigated the process of supramolecular polymerization that comprises synthesis of long molecular chains (polymers) made of multiple repeating units (mers). Synthetic polymers are indispensable in everyday life and we can hardly imagine how would our world look like without polymers. Materials such as polyethylene, polypropylene or poly(ethylene terephthalate) are commonly used every day from plastic to car bumpers. Above mentioned polymers are covalent polymers (or macromolecules). The bonds between consequtive mers are of covalent nature, thus the atoms are connected via shared electron pair. In Eindhoven we studied supramolecular polymers – structures based on non-covalent bonds between mers, e.g. hydrogen bonds. The main difference between covalent and non-covalent polymers is the reversibility of the synthesis of the latter. With use of appropriate external stimuli such as temperature, solvent or another chemical we can reverse polymerization to retrieve the monomers back. On the contrary, covalent polymers (excluding a very specific class of dynamic polymers) are non-reversible thus their structure cannot be modified. Supramolecular polymers can form variety of secondary structures depending on the specific non-covalent interactions employed to build the polymer scaffold. For example, when hydrogen-bonding groups in the monomer are arranged with appropriate symmetry, it polymerizes into a helical structure. Helices are inherently chiral structures that can be either right-(P) or left-handed (M). Helical bias of supramolecular polymer can be controlled by presence of chiral elements in the monomer structure or chirality of a solvent used for polymerization. Interestingly, some supramolecular polymers exhibit many properties characteristic for crystals. For example, the polymerization can proceed following nucleation- elongation mechanism observed in crystals. An important feature characteristic for crystals and for many helical supramolecular polymers is cooperativity – synergistic additivity of multiple interactions within the supramolecular structure. In a cooperative system, all non-covalent interactions influence each other and add up energetically. This gives rise to many non-linear phenomena observed also in biomolecules.
Covalent polymers such as poly (ethylene terephthalate) (on the left) consist of monomers (terephthalic acid and ethylene glycol) linked via covalent bond. Supramolecular polymers are based on non-covalent bonds (e.g. hydrogen bonds) between consecutive mers. Cyclic (aromatic) compounds functionalized with amide groups show ability to form stable hydrogen bonds. Appropriate symmetry in the monomer structure (here: rotational symmetry C3) facilitates formation of a helical structure.
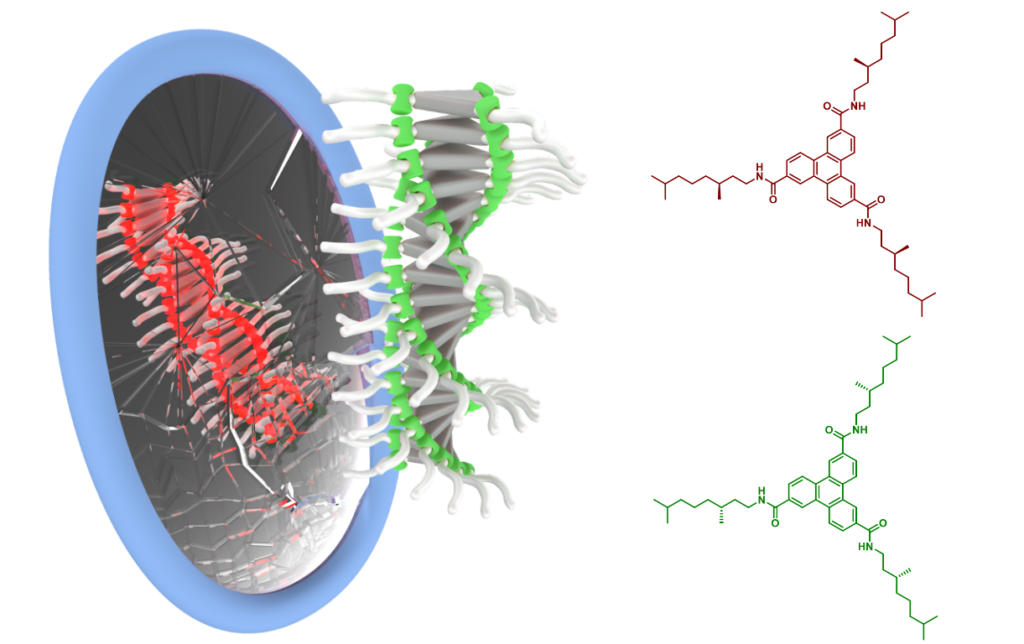
As mentioned earlier in the text, we controlled the helical bias of supramolecular polymers by incorporating chirality in the monomer structure. However, owing to the cooperativity phenomena, we did not need to have monomer pool consisting of exclusively chiral monomers to yield full helical bias. It is enough to use only few percent of a chiral monomer (A’) in a pool on achiral monomers (B) to bias the helicity of the whole system into helicity preferred by A’. In scientific literature this phenomenon is described as “sergeants and soldiers”.
Everything I described so far in the text is well understood and so my results were not surprising to anyone. However, one Friday evening I was trying to come up with a very original idea for my last chapter and I indeed came up with something completely new. With analogy to the “sergeants and soldiers” experiment, I envisioned to run a rebellion – the situation where achiral monomers B (forced by chiral solvent) would dictate the helicity to chiral monomers A’. Achiral monomers B when dissolved in (S)-1-chloro-2-methylbutane polymerize exclusively into P-helices, while chiral monomers A’ (enantiomer R) prefer M helicity. By copolymerization of A’ with B in the chiral solvent, I obtained exclusively P-helices which supported my thesis of rebellion taking place. However, when I investigated more in detail the helicity of the copolymers as function of temperature, I observed helix inversion transition, which I have not seen before in any other solvent.
Broken symmetry
Given the complexity of the system, I realized that I have to first understand well supramolecular polymerization of both enantiomers A and A’ of the chiral monomer in the chiral solvent of S-configuration separately. I saw helix inversion transition occurring upon cooling in case of both enantiomers, depending on the configuration, P to M (A’, enantiomer R) or M to P (A, enantiomer S). It would be absolutely normal if I have not observed different helix inversion temperatures for A and A’ in S-chiral solvent! I can very well remember reaction of my PhD advisor Bert, when he has seen the curves for the first time. He was absolutely shocked and on that moment I realized that we just stepped into something big. My fellow PhD student, Mathijs Mabesoone created a thermodynamic model that mathematically described the system and with its help we draw some surprising conclusions. The results of our experiments unambiguously confirmed that the chiral solvent broke the mirror symmetry between enantiomers, which made the truthfulness of van ‘t Hoff thesis indisputable. After careful analysis of previous experiments we realized, why no one before us (including van ‘t Hoff himself) could achieve the goal. To the best of our knowledge, all the chemists trying to tackle this problem before us studied solubility of enantiomers in a chiral solvent. Not surprisingly, they have not observed any detectable difference in this property for S and R. Solubility is a monomer feature and it is about monomer-solvent interaction. It this case, there is no discussion about additivity of the interactions. Therefore even if there is difference in solubility between enantiomers S and R of solute molecule in S-solvent, it is too small to be experimentally detectable. However, in our experiments we studied polymers, where owing to the cooperativity phenomenon, we observe additivity of the interactions. In effect, even minimal difference in energy is multiplied by the chain length (a factor of hundreds of thousands) and gives measurable difference between enantiomers.
Helical supramolecular polymers can adopt a form of exclusively left- or right-handed helix when there is chirality in the monomer or solvent structure. In an achiral solvent, helices P and M are enantiomers such as building blocks A and A’. Therefore the helices P and M exhibit identical chemical properties. In chiral solvent, the mirror symmetry is broken and the polymers behave as diastereomers – they have different chemical properties. The illustration above presents broken mirror symmetry.
By using of the model created by Mathijs, we could dive into the thermodynamics of the mirror symmetry breaking. We concluded that the energy difference (expressed in terms of Gibbs free energy, ΔG) between the enantiomers in chiral solvent originates exclusively from entropy ΔS and has little to do with enthalpy ΔH.
The great unknown
Our later experiments showed that the observations described above can originate from non- linear phenomena caused by the solvent. In other words, we should see the solvent as a vector field rather than just a set of molecules. Unfortunately, I will not dive into this discussion. As time of my PhD came shortly after to the end, I had to quit the group and abandon any further experiments thus the whole fascinating journey into the nooks and crannies of chemistry. Nevertheless, still after few years I hope that one day a great chemist will finally answer the question – what a solvent is, actually? It is absolutely contradictory that solvents are inevitable in almost every chemical reaction, yet we still know so little about them.
Based on: Nature Chemistry, 13, 200–207 (2021)
Marcin Ślęczkowski