Nutrition research
Nutrition science is a relatively new area of research. Recent nutrition studies try to combine epidemiology (studies on the population level) with a molecular approach (studies at a molecular level, including cells and genetics) (1). This type of approach allows for studying inter-individual differences including a significant number of participants to detect small but significant differences, as well as to confirm underlying mechanisms responsible for these differences (e.g. genetic susceptibility to a specific disease).
Moreover, recent advances in technology allowed us to get a better insight into the pathophysiological processes responsible for the development of dietary-related diseases. These findings highlighted new treatment targets such as the gut microbiota, thanks to microbial DNA sequencing or nutrigenomics, thanks to DNA sequencing of the whole human genome (genetic information), known as Genome-Wide Association Studies (GWAS).
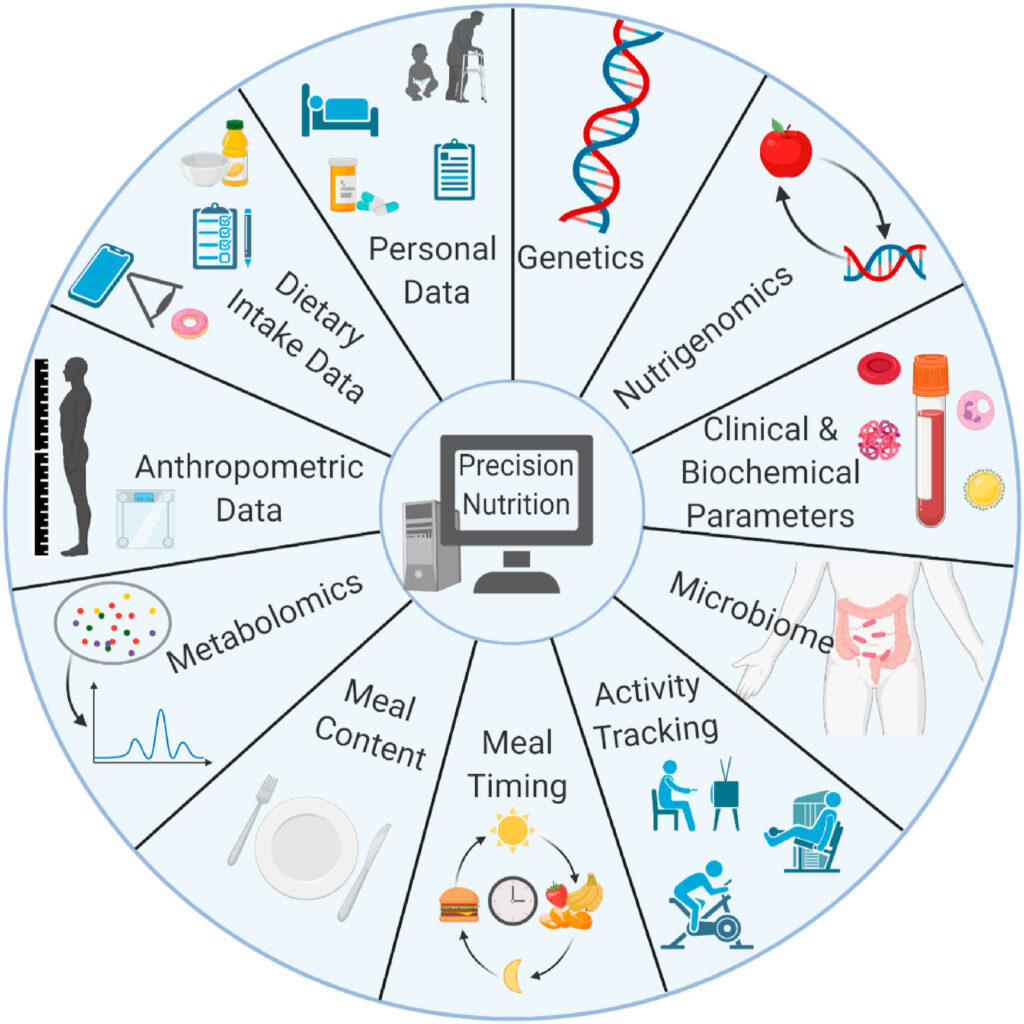
Precision medicine and personalised nutrition
Thanks to the development of high-throughput sequencing and generating big data with less time and costs than ever before, there is a development of the precision medicine field in the research with the usage of -omic data (genomics, transcriptomics, metabolomics, microbiomics). This has led to the concept of precision nutrition based on individualised dietary recommendations which could support public health nutrition guidelines. Personalised nutrition involves traditional methods of measures such as clinical and biochemical parameters or anthropometry. There are also new biomarker tools at the stage of development such as the identification of specific metabolites from the urine for the detection of the diet quality of an individual (3) but also nutrigenomics and microbiome testing.
.
Dietary impact
We know that genetic predisposition is an important contributor to the mortality of noncommunicable diseases such as cardiovascular disease, diabetes, type II and cancers. On the other hand, nutrients influence the metabolic programming of cells. Globally, one in five deaths is associated with a poor diet (a diet low in grains, fruits and high in sodium) which is more deaths than from smoking (4). Combining both genetic and environmental influences, we can explore the gene-environment interactions of nutrients.
Nutrigenomics vs nutrigenetics
Nutritional genomics research studies the interactions between bioactive components from food and the genome, which includes nutrigenetics and nutrigenomics areas. This means that food can affect people differently with two underlying biochemical mechanisms:
- influence of genetic information on how humans respond to certain foods, known as nutrigenetics
- food influence on genetics (nutrients affecting our gene expression), known as nutrigenomics
Nutrigenetics in practice
It is not a novel idea that inherited genes can impact our response to foods, making our dietary needs very individual. As an example, a genetic disease called phenylketonuria affects individuals who must avoid foods containing the amino acid called phenylalanine. It cannot be metabolised appropriately due to the mutation in the gene coding enzyme responsible for its metabolism called Phenylalanine hydroxylase (PAH) (5). Phenylalanine can be found in products such as meat, fish, eggs, cheese, beans and nuts. If eaten, it accumulates in the body and can negatively affect the cognitive function of children, leading to learning disabilities. These serious consequences can be avoided by providing newborn screening tests in the first days of life, present in Poland.
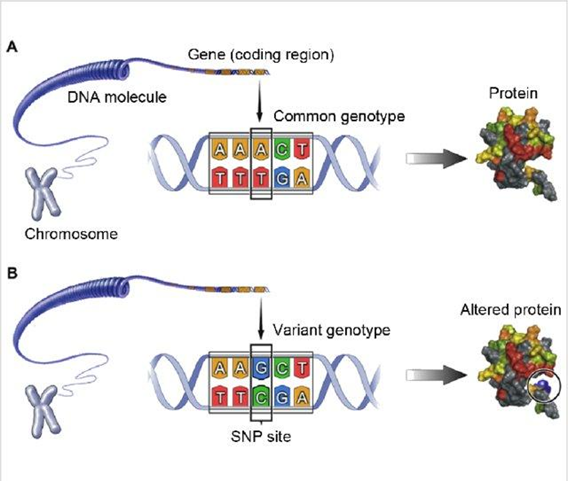
Phenylketonuria is caused by a change (mutation) in a single gene, whereas today’s research focuses on changes in a specific variant of nucleotides. The difference is that a gene is a bigger fragment of a distinct sequence of nucleotides forming part of a chromosome. That means the nucleotide is a smaller unit which forms a gene and its change will impact the gene variant. Having a specific gene variant, known as Single Nucleotide Polymorphism (SNP) can also influence nutrient metabolism. This is the case for lactose intolerance, which makes people unable to digest milk products. This is because the gene encoding lactase (the enzyme which breaks down lactose), is normally ‘turned off’ after weaning. It is estimated that most adults have lactose intolerance (in Poland the prevalence is at the level of 43%, whereas the estimated global prevalence of lactose malabsorption is 74%) (6).
However, about 11,000 years ago a polymorphism in a single DNA nucleotide appeared among northern Europeans which resulted in the continued expression of the lactase gene into adulthood (5). From an evolutionary perspective, this was advantageous as people in regions with short growing seasons had a new variant of nucleotide and could digest the milk products, which is a rich source of nutrients including sugar like lactose (disaccharide build up from galactose and glucose).
The other example can be the methylenetetrahydrofolate reductase (MTHFR) gene. It impacts the enzyme which plays a role in folate metabolism by converting it to the active form of methyl folate. People with some variations of the MTHFR gene may be at risk of folate deficiency, regardless of their dietary intake and may require the supplementation of methyl folate. More interestingly, combining research studies involving genome and microbiome, it was found that a variant of the FUT2 gene is associated with both predisposition to decreased levels of vitamin B12 and changes in the attachment of the bacterium Helicobacter pylori to the gastric mucosa, affecting vitamin B12 absorption (7).
.
Nutrigenomics in practice
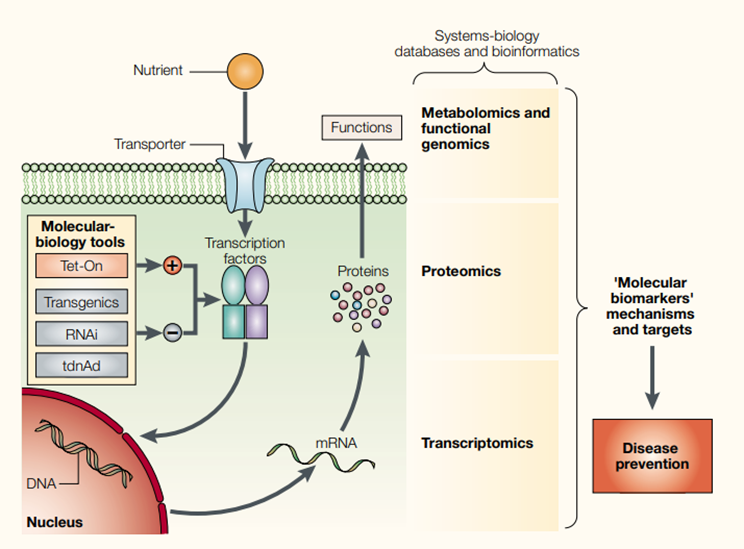
Nutrients can be called ‘dietary signatures’ as they are detected by the cellular sensor systems which influence gene and protein expression and consequently, metabolite production. They can affect gene expression directly or indirectly by acting as ligands for transcription factor receptors or be metabolised by primary or secondary metabolic pathways (altering concentrations of substrates or intermediates, affecting signalling pathways). Moreover, the role of epigenetics processes should not be forgotten- the response to food components depends on DNA methylation influencing gene expression. There are two strategies in research to identify relevant nutrigenomics pathways which are hypothesis-driven with specific genes and their expression influenced by nutrients, as well as very recent systems biology approaches with gene, protein and metabolite signatures that are associated with specific nutrients or dietary patterns.
Various nutrients including antioxidants are known to enhance DNA repair and reduce oxidative DNA damage. Those mechanisms play a role in non-communicable diseases including diabetes, cardiovascular diseases and metabolic syndrome. As an example, transcription factor 7-like 2 (TCF7L2) polymorphism is strongly associated with type 2 diabetes (9). One study showed that the Mediterranean diet reduces the adverse effects of the gene variant on cardiovascular risk factors (10). The Mediterranean diet is known for being rich in fresh vegetables, fruit, whole grains, fish and most importantly, olive oil.
Resveratrol is a natural antioxidant from the phenol group which can be found in several plants such as peanuts, grapes, raspberries, and vegetables (11). Resveratrol was found to be an activator of the SIRT gene and it has been used as a treatment for diabetes by normalising hyperglycaemia and improving insulin sensitivity (12).
Finally, there are a couple of human studies confirming the positive effect of omega-3 polyunsaturated fatty acids. It was shown that 8-week fish oil supplementation increased PPARγ in subjects with type 2 diabetes in comparison with the placebo group, improving lipid metabolism and energy homeostasis (13). PPARγ is a transcription factor that prevents pro-inflammatory cytokines release (13).
Dietary and genetic measures in research
Nutrition research including Nutrigenetics and Nutrigenomics is a relatively new area of research. The development of this field is important as it may enable medical practitioners and dieticians to provide more personalised care of patients in Poland, diagnosed with dietary-related diseases. There is a need for more studies to implement precision medicine tools into practice. Not only is there a need for mathematical modelling of multiple genetic effects, but there is also a potential in incorporating and combining different types of -omic data together (multi-omic studies) and exploring different dietary patterns and nutrients effect in specific conditions and groups of interest. We can see in the industry the development of companies such as Zoe for personalised nutrition and other companies including microbiome and genetic testing. However, we need to take into account that the scientific evidence behind these tools for usage in clinical practice is still very limited. We need more conclusions from future research studies incorporating big data and machine learning. Moreover, the development of statistical frameworks such as Mendelian Randomisation for proving causality in epidemiology studies would facilitate future findings. Development of this field may enable medical practitioners and dieticians to provide more personalised care of patients in Poland, diagnosed with dietary-related diseases.
References
- Müller M, Kersten S. Nutrigenomics: goals and strategies. Nat Rev Genet [Internet]. 2003 Apr 1 [cited 2023 Apr 5];4(4):315–22. Available from: https://pubmed.ncbi.nlm.nih.gov/12671662/
- Kirk D, Catal C, Tekinerdogan B. Precision nutrition: A systematic literature review. Comput Biol Med. 2021 Jun 1;133:104365.
- Garcia-Perez I, Posma JM, Chambers ES, Mathers JC, Draper J, Beckmann M, et al. RETRACTED ARTICLE: Dietary metabotype modelling predicts individual responses to dietary interventions. Nature Food 2020 1:6 [Internet]. 2020 Jun 17 [cited 2023 Apr 5];1(6):355–64. Available from: https://www.nature.com/articles/s43016-020-0092-z
- Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet [Internet]. 2019 May 11 [cited 2023 Apr 5];393(10184):1958–72. Available from: http://www.thelancet.com/article/S0140673619300418/fulltext
- Neeha VS, Kinth P. Nutrigenomics research: a review. J Food Sci Technol [Internet]. 2013 Jun 1 [cited 2023 Apr 5];50(3):415. Available from: /pmc/articles/PMC3602567/
- Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol [Internet]. 2017 Oct 1 [cited 2023 Apr 5];2(10):738–46. Available from: http://www.thelancet.com/article/S2468125317301541/fulltext
- Velkova A, Diaz JEL, Pangilinan F, Molloy AM, Mills JL, Shane B, et al. The FUT2 secretor variant p.Trp154Ter influences serum vitamin B12 concentration via holo-haptocorrin, but not holo-transcobalamin, and is associated with haptocorrin glycosylation. Hum Mol Genet [Internet]. 2017 Dec 12 [cited 2023 Apr 5];26(24):4975. Available from: /pmc/articles/PMC5886113/
- Iglesias MS, Grzelczak M. Using gold nanoparticles to detect single-nucleotide polymorphisms: toward liquid biopsy. Beilstein Journal of Nanotechnology. 2020 Jan 31;11:263–84.
- Felisbino K, Granzotti JG, Bello-Santos L, Guiloski IC. Nutrigenomics in Regulating the Expression of Genes Related to Type 2 Diabetes Mellitus. Front Physiol. 2021 Jul 21;12:1109.
- Corella D, Carrasco P, Sorlí J V., Estruch R, Rico-Sanz J, Martínez-González MÁ, et al. Mediterranean diet reduces the adverse effect of the TCF7L2-rs7903146 polymorphism on cardiovascular risk factors and stroke incidence: a randomized controlled trial in a high-cardiovascular-risk population. Diabetes Care [Internet]. 2013 Nov [cited 2023 Apr 5];36(11):3803–11. Available from: https://pubmed.ncbi.nlm.nih.gov/23942586/
- Chao SC, Chen YJ, Huang KH, Kuo KL, Yang TH, Huang KY, et al. Induction of sirtuin-1 signaling by resveratrol induces human chondrosarcoma cell apoptosis and exhibits antitumor activity. Scientific Reports 2017 7:1 [Internet]. 2017 Jun 9 [cited 2023 Apr 5];7(1):1–11. Available from: https://www.nature.com/articles/s41598-017-03635-7
- Goh KP, Lee HY, Lau DP, Supaat W, Chan YH, Koh AFY. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int J Sport Nutr Exerc Metab [Internet]. 2014 [cited 2023 Apr 5];24(1):2–13. Available from: https://pubmed.ncbi.nlm.nih.gov/23918588/
- Bordoni L, Petracci I, Zhao F, Min W, Pierella E, Assmann TS, et al. Nutrigenomics of dietary lipids. Antioxidants [Internet]. 2021 Jul 1 [cited 2023 Apr 5];10(7). Available from: /pmc/articles/PMC8300813/
Martyna Kościuszko


